Clostridium difficile Infection
For the past four years I have been a PhD thesis committee member for a graduate student. Last week she gave her final thesis defense seminar, describing her research into the pathogenesis of Clostridium difficile infection (CDI). I’m pleased and proud to say that she performed wonderfully, presenting a beautiful synopsis of her research, and additionally dealt extremely well with our questions from the committee afterwards. (Congratulations Jenny!)
Interestingly, I overheard a conversation at the checkout in my local pharmacy just an hour later. One gentleman was telling the cashier how his wife had recently been taking antibiotics in hospital, and had subsequently developed a terrible diarrhea-related illness.
Needless to say, it turned out that she had CDI. Quite bizarre that I had just spent a whole afternoon discussing this very disease! So it seems like a fitting opportunity to briefly mention this important condition.
Clostridium difficile
Clostridium difficile is a gram positive, spore-forming, anaerobic, toxin-producing bacterial organism. Two of its toxins in particular, TcdA and TcdB, are responsible for most of the important, pathologic changes associated with its infection.
Clostridium difficile Infection (CDI)
This agent causes a spectrum of intestinal pathology in people that ranges from diarrhea to life-threatening necrotizing colitis. The incidence of CDI has certainly risen dramatically over the past 30 years or so, specifically in association with increasing use of antibiotics in healthcare. Interestingly, CDI is the most common infection acquired by hospitalized patients, and its incidence seems to have surpassed that of Methicillin-resistant Staphylococcus aureus (MRSA) infections in hospitals. Although elderly patients are at greatest risk of infection, the incidence of disease in younger patients is actually increasing.
Pathophysiology
The inciting event for Clostridium difficile-associated disease is disruption of the normal microflora of the bowel – typically due to broad spectrum antibiotic therapy. Clostridium difficile spores can persist in the environment, and are ingested by patients. Typically they cause no problem, but following disruption of the bowel’s microflora in these antibiotic-treated patients, their ingestion subsequently allows Clostridium difficile to colonize the colon. Host-associated factors (such as age, immune status, concurrent disease status) now determine what happens next.
Either a patient will become an asymptomatic carrier, or can develop disease. This disease can range from mild diarrhea to potentially fatal pseudomembranous colitis. This colitis arises as a result of the action of toxins TcdA and TcdB.
TcdA especially seems to have enterotoxic activity. It activates inflammatory cells, stimulating them to produce inflammatory mediators that lead to fluid secretion and increased intestinal mucosal permeability. As inflammation progresses, the colonic mucosa becomes ulcerated, with accumulation of inflammatory cells and necrotic cell debris on the colonic surface. This produces the classic pseudomembrane overlying the mucosal surface. Both TcdA and TcdB additionally have cytotoxic activity that contributes to the colitis of CDI.
Most cases of CDI are known to occur between 4-9 days of antibiotic therapy, and a patient’s risk of developing diarrhea due to CDI doubles with more than 3 days of antibiotic therapy. This organism is responsible for approximately 25% of cases of antibiotic-associated diarrhea, and up to 75% of cases of antibiotic-associated colitis in people. Current estimates suggest that there are approximately 500,000 cases of CDI in the US annually, and up to 20,000 of these cases result in death.
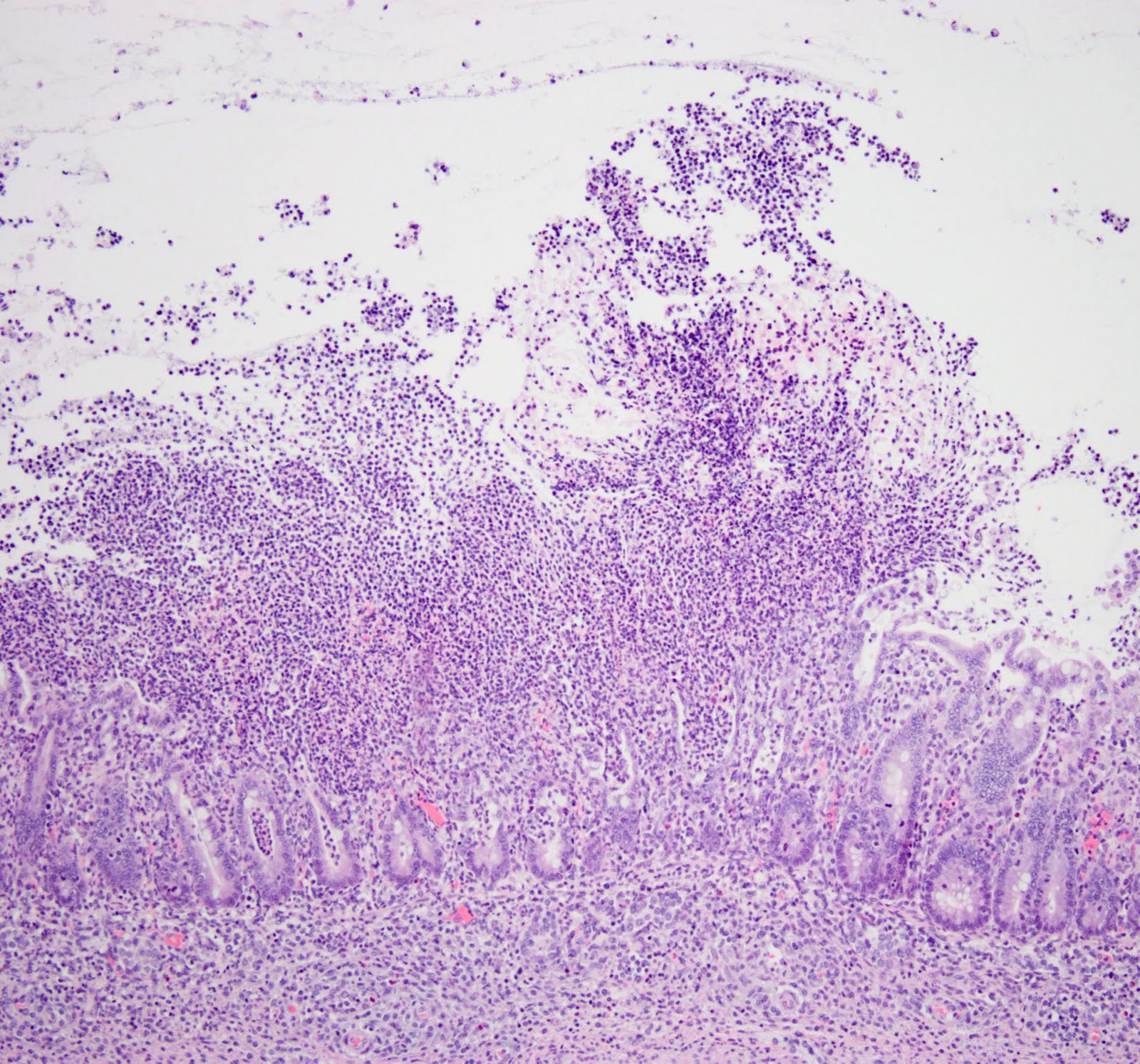 |
| A photomicrograph showing histopathologic changes in the colon: Severe inflammation and ulceration of the colon due to Clostridium difficile infection |
So clearly CDI is an important problem amongst hospitalized patients. Its increasing incidence, combined with the associated financial implications to the healthcare system, certainly contribute to the fact that this condition is being widely researched in the scientific community.